Decitabine CAS NO 2353-33-5 Inquire about Decitabine
Tecoland supplies Decitabine bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Decitabine is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Decitabine?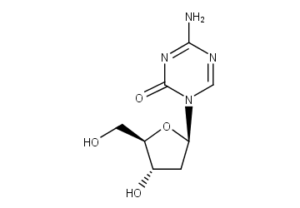
Decitabine (trade name Dacogen), or 5-aza-2′-deoxycytidine, is a cytidine analog. Decitabine is indicated for treatment of patients with myelodysplastic syndrome (MDS). It is a chemical analogue of cytidine, a nucleoside present in DNA. Cells in the presence of Decitabine incorporate it into DNA during replication. The incorporation of Decitabine into DNA inhibits methyltransferase thereby causing demethylation in that sequence. This adversely affects the way that cell regulatory proteins are able to bind to the DNA substrate.
What is the mechanism of action?
It is a hypomethylating agent. It hypomethylates DNA by inhibiting DNA methyltransferase.
It functions in a similar manner to Azacitidine, although Decitabine can only be incorporated into DNA strands while Azacitidine can be incorporated into both DNA and RNA chains.
Clinical uses
Decitabine is indicated for the treatment of myelodysplastic syndromes (MDS) including previously treated and untreated, de novo and secondary MDS of all French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia) and Intermediate-1, Intermediate-2, and High-Risk International Prognostic Scoring System groups. In patients with renal insufficiency, Batty and colleagues reported the first case series on the feasibility of therapy with hypomethylating agents in patients with renal insufficiency.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.