Carboplatin CAS NO 41575-94-4 Inquire about Carboplatin
Tecoland supplies Carboplatin bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Carboplatin is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Carboplatin?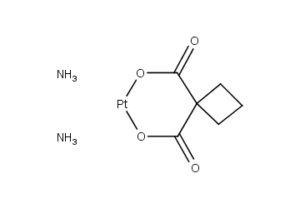
Carboplatin is a chemotherapy drug used against some forms of cancer (mainly ovarian carcinoma, lung, head and neck cancers). It was introduced in the late 1980s and has since gained popularity in clinical treatment due to its vastly reduced side-effects compared to its parent compound Cisplatin. Cisplatin and Carboplatin, as well as Oxaliplatin, interact with DNA, akin to the mechanism of alkylating agents.
Chemistry of Carboplatin
Carboplatin differs from Cisplatin in that it has a bidentate dicarboxylate (CBDCA) ligand as its leaving group instead of the more labile chloride ligands. It exhibits lower reactivity and slower DNA binding kinetics, though it forms the same reaction products in vitro at equivalent doses with Cisplatin. Unlike Cisplatin, Carboplatin may be susceptible to alternative mechanisms. Some results show that Cisplatin and Carboplatin cause different morphological changes in MCF-7 cell lines while exerting their cytotoxic behaviour. The diminished reactivity limits protein-Carboplatin complexes, which are excreted. The lower excretion rate of Carboplatin means that more is retained in the body, and hence its effects are longer lasting (a retention half-life of 30 hours for Carboplatin, compared to 1.5-3.6 hours in the case of Cisplatin).
What is the mechanism of action?
Two theories exist to explain the molecular mechanism of action of Carboplatin with DNA:
- Aquation, or the like-Cisplatin hypothesis.
- Activation, or the unlike-Cisplatin hypothesis.
The former is more accepted owing to the similarity of the leaving groups with its predecessor Cisplatin, while the latter hypothesis envisages a biological activation mechanism to release the active Pt2+ species.
What are the side effects of Carboplatin?
Relative to Cisplatin, the greatest benefit of Carboplatin is its reduced side effects, particularly the elimination of nephrotoxic effects. Nausea and vomiting are less severe and more easily controlled. Carboplatin has also proven effective in some strains of cancer that may not be susceptible to Cisplatin, including germ-line cell, small and non-small cell lung, ovary, and bladder cancers, as well as acute leukemia.
The main drawback of Carboplatin is its myelosuppressive effect. This causes the blood cell and platelet output of bone marrow in the body to decrease quite dramatically, sometimes as low as 10% of its usual production levels. The nadir of this myelosuppression usually occurs 21-28 days after the first treatment, after which the blood cell and platelet levels in the blood begin to stabilize, often coming close to its pre-Carboplatin levels. This decrease in white blood cells (neutropenia) can cause complications, and is sometimes treated with drugs like filgrastim. The most notable complication of neutropenia is increased probability of infection by opportunistic organisms, which necessitates readmission to hospital and treatment with antibiotics.
Relative to Cisplatin, the potency of Carboplatin is less potent: depending on the strain of cancer, Carboplatin may only be 1/8 to 1/45 as effective. The clinical standard of dosage of Carboplatin is usually a 4:1 ratio compared to Cisplatin; that is, for a dose that usually requires a particular dose of Cisplatin, four times more Carboplatin is needed to achieve the same effectiveness. The stable property of Carboplatin is a mixed blessing: once uptake of the drug occurs, its retention half-life is considerably longer than Cisplatin, but it is also this inertness that causes Carboplatin to go right through the human body, and up to 90% of the Carboplatin given can be recovered in urine.
The effectiveness of Carboplatin can be increased by first incubating Carboplatin in a sodium chloride (NaCl) solution. After 24 hours, an analysis was performed on the solution by separating the compounds by thin-layer chromatography (TLC). The TLC isolated Cisplatin, Carboplatin, and several platinum by-products in the solution. Numerous trials were conducted, and the trend showed that the survival rate of E. coli dropped dramatically as the molarity of the NaCl incubating solution increased. The treated E. coli also showed decreased amounts of alkaline phosphatase, a protein indicator of cellular size. This suggests that as this incubated Carboplatin solution was administered to cells, they began to shrink and eventually die; apparently by the same mechanism that Cisplatin works.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.