Capecitabine CAS NO 154361-50-9 Inquire about Capecitabine
Tecoland supplies Capecitabine bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Capecitabine is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Capecitabine?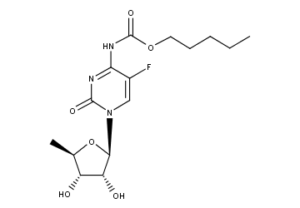
Capecitabine (Xeloda, Roche) is an orally-administered chemotherapeutic agent used in the treatment of metastatic breast and colorectal cancers. Capecitabine is a prodrug, that is enzymatically converted to 5-fluorouracil in the tumor, where it inhibits DNA synthesis and slows growth of tumor tissue. The activation of Capecitabine follows a pathway with three enzymatic steps and two intermediary metabolites, 5′-deoxy-5-fluorocytidine (5′-DFCR) and 5′-deoxy-5-fluorouridine (5′-DFUR), to form 5-fluorouracil.
Mechanism of action of Capecitabine
Capecitabine is a prodrug, that is enzymatically converted to 5-fluorouracil in the tumor, where it inhibits DNA synthesis and slows growth of tumor tissue.
May interact with warfarin and increase bleeding risk.
May inhibit cytochrome CYP2C9 enzyme, and therefore increase levels of substrates such as phenytoin and other substrates of CYP2C9.
The concomitant use of leucovorin is not recommended. Manufacturer’s warning In a controlled study, leucovorin increased the toxicity of Capecitabine without any apparent advantage in response rate.
How to dose Capecitabine?
The usual starting dose is 2,500 mg/m2/day in two divided doses, 12 hours apart. One cycle includes two weeks of treatment followed by one week without treatment. Cycles can be repeated every three weeks.
Dose adjustments
For mild renal dysfunction (creatinine clearance 30-50 mL/min), it is recommended to reduce dose by 25%.
For severe renal dysfunction (creatinine clearance.
There is no recommendation for hepatic dysfunction.
For elderly patients, lower doses may be required due to higher incidences of serious adverse reactions.
Patients with Dihydropyrimidine dehydrogenase deficiency (a.k.a. DPD deficiency), a pharmacogenetic syndrome affecting Capecitabine detoxification process in the liver, should have their dosage tailored.
What are the side effects?
Potential major adverse reactions include:
Cardiovascular: EKG changes, myocardial infarction, angina (these may be more common in patients with pre-existing coronary artery disease)
Dermatological: Hand-foot syndrome (numbness, tingling, pain, redness, or blistering of the palms of the hands and soles of the feet). This can lead to the disappearance of fingerprints in some patients.
Gastrointestinal: Diarrhea (sometimes severe), nausea, and stomatitis have occurred. Octreotide has been studied as an anti-diarrheal in cases of refractory diarrhea associated with Capecitabine use.
Hematological: Neutropenia, anemia, and thrombocytopenia.
Hepatic: Hyperbilirubinemia
Capecitabine Storage
Store at 25C (77F); excursions permitted to 15 to 30C (59 to 86F).
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.