Anidulafungin CAS NO 166663-25-8 Inquire about Anidulafungin
Tecoland supplies Anidulafungin bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Anidulafungin is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects. We supply from small R&D to commercial Quantity.
What is Anidulafungin?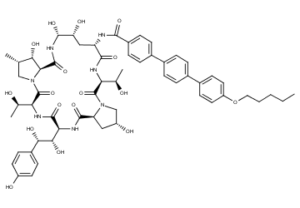
Anidulafungin is an antifungal medicine that is used to treat candida (yeast) infections in the blood or the stomach in adults and children at least 1 month old.
Anidulafungin is also used to treat candida infections of the esophagus only in adults.
Anidulafungin may also be used for purposes not listed in this medication guide.
Important Information
Follow all directions on your medicine label and package. Tell each of your healthcare providers about all your medical conditions, allergies, and all medicines you use.
Before taking this medicine
You should not use anidulafungin if:
- you are allergic to anidulafungin or similar medicines such as caspofungin or micafungin; or
- you have hereditary fructose intolerance, a condition that makes it hard for you to digest fructose sugars (such as in certain fruits or juices).
Tell your doctor if you have ever had liver disease.
Tell your doctor if you are pregnant or breastfeeding.
How is anidulafungin given?
Follow all directions on your prescription label and read all medication guides or instruction sheets. Use the medicine exactly as directed.
Anidulafungin is given as an infusion into a vein. A healthcare provider will give your first dose and may teach you how to properly use the medication by yourself.
Anidulafungin must be mixed with a liquid (diluent) and then further diluted in an IV bag before using it. Read and carefully follow any Instructions for Use provided with your medicine. Ask your doctor or pharmacist if you don’t understand all instructions.
Prepare an injection only when you are ready to give it. Do not use if the medicine has changed colors or has particles in it. Call your pharmacist for new medicine.
This medicine must be given slowly, and the infusion can take at least 45 minutes to 3 hours to complete.
You may need to use an infusion pump or special syringe when giving anidulafungin to a child. Follow your doctor’s instructions very carefully.
Anidulafungin is sometimes given for up to 7 days after your symptoms clear up, or up to 14 days after lab tests show that the infection has cleared. Use this medicine for the full prescribed length of time. Anidulafungin will not treat a viral infection such as the flu or a common cold.
You may need frequent blood tests to check your liver function.
Store unused vials in a refrigerator. Do not freeze.
After mixing the diluent into a vial, you may keep the vial at room temperature for up to 24 hours before you further dilute the mixture in an IV bag and give the injection.
The final mixed solution in the IV bag may be kept at room temperature for up to 48 hours. Do not freeze.
Each vial (bottle) is for one use only. Throw it away after one use, even if there is still medicine left inside.
After treatment with anidulafungin, you may be given other antifungal medication to keep your esophageal candida infection from coming back. Keep using this medicine for as long as your doctor has prescribed.
Do not use anidulafungin diluted solution if it has changed colors or has particles in it. Call your pharmacist for new medication.
Use a needle and syringe only once and then place them in a puncture-proof “sharps” container. Follow state or local laws about how to dispose of this container. Keep it out of the reach of children and pets.
What happens if I miss a dose?
Call your doctor for instructions if you miss a dose.
What happens if I overdose?
Seek emergency medical attention or call the Poison Help line at 1-800-222-1222.
What should I avoid while using anidulafungin?
Follow your doctor’s instructions about any restrictions on food, beverages, or activity.
Anidulafungin side effects
Get emergency medical help if you have signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
Some side effects may occur during the injection. Tell your caregiver right away if you feel light-headed, itchy, warm, tingly, sweaty, or if you have chest tightness or trouble breathing.
Call your doctor at once if you have:
- a light-headed feeling, like you might pass out;
- trouble breathing;
- little or no urination, dark urine, pain or burning when you urinate;
- stomach pain and bloating;
- easy bruising, unusual bleeding, purple or red spots under the skin;
- low potassium–leg cramps, constipation, irregular heartbeats, fluttering in your chest, increased thirst or urination, numbness or tingling, muscle weakness or limp feeling;
- low red blood cells (anemia)–pale skin, unusual tiredness, feeling light-headed or short of breath, cold hands and feet; o
- (in a baby) severe drowsiness, fussiness, feeding problems, or vomiting.
Common side effects may include:
- abnormal liver function tests;
- low blood sugar (hunger, sweating, irritability, dizziness, feeling shaky);
- sore or white patches in your mouth or throat;
- low blood pressure, feeling light-headed;
- anemia, bruising or bleeding, nosebleed;
- fever;
- stomach pain, indigestion, nausea, vomiting, diarrhea;
- low potassium;
- headache, trouble sleeping; or
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Anidulafungin dosing information
Usual Adult Dose for Candidemia:
Initial dose: 200 mg IV as a single loading dose on Day 1
Maintenance dose: 100 mg IV once a day thereafter
Comments:
-Duration of therapy should be based on patient’s clinical response.
-In general, antifungal therapy should continue for at least 14 days after the last positive culture.
Use: For the treatment of candidemia and the following Candida infections: intraabdominal abscess and peritonitis
Usual Adult Dose for Esophageal Candidiasis:
Initial dose: 100 mg IV as a single loading dose on Day 1
Maintenance dose: 50 mg IV once a day thereafter
Duration of therapy: At least 14 days and at least 7 days after resolution of symptoms
Comments:
-Duration of therapy should be based on patient’s clinical response.
-Due to risk of relapse in HIV-infected patients, suppressive antifungal therapy may be considered after a course of treatment.
Use: For the treatment of esophageal candidiasis
Usual Pediatric Dose for Candidemia:
1 month or older:
-Initial dose: 3 mg/kg IV as a single loading dose on Day 1
—Maximum dose: 200 mg/dose
-Maintenance dose: 1.5 mg/kg IV once a day thereafter
—Maximum dose: 100 mg/dose
Comments:
-Overall, antifungal therapy should continue for at least 14 days after the last positive culture.
Use: For the treatment of candidemia and the following Candida infections: intraabdominal abscess and peritonitis
What other drugs will affect anidulafungin?
Other drugs may affect anidulafungin, including prescription and over-the-counter medicines, vitamins, and herbal products. Tell your doctor about all your current medicines and any medicine you start or stop using.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.
External Link:
Anidulafungin. (n.d.). Drugs.Com. https://www.drugs.com/mtm/anidulafungin.html