Rocuronium Bromide CAS NO 119302-91-9 Inquire about Rocuronium Bromide
Tecoland supplies Rocuronium Bromide bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Rocuronium Bromide is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Rocuronium Bromide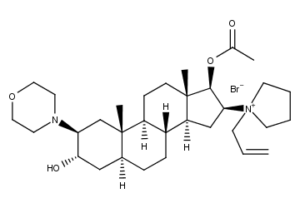
Rocuronium bromide (brand names Zemuron, Esmeron) is an aminosteroid non-depolarizing neuromuscular blocker or muscle relaxant used in modern anaesthesia to facilitate tracheal intubation by providing skeletal muscle relaxation, most commonly required for surgery or mechanical ventilation. It is used for standard endotracheal intubation, as well as for rapid sequence induction (RSI).
Pharmacology
Mechanism of action
It was designed to be a weaker antagonist at the neuromuscular junction than pancuronium; hence its monoquaternary structure and its having an allyl group and a pyrrolidine group attached to the D ring quaternary nitrogen atom. Rocuronium has a rapid onset and intermediate duration of action.
There is considered to be a risk of allergic reaction to the drug in some patients (particularly those with asthma), but a similar incidence of allergic reactions has been observed by using other members of the same drug class (non-depolarizing neuromuscular blocking drugs).
The γ-cyclodextrin derivative sugammadex (trade name Bridion) has been recently introduced as a novel agent to reverse the action of rocuronium. Sugammadex has been in use since 2009 in many European countries; however, it was turned down for approval twice by the US FDA due to concerns over allergic reactions and bleeding, but finally approved the medication for use during surgical procedures in the United States on December 15, 2015. Neostigmine can also be used as a reversal agent of rocuronium but is not as effective as sugammadex. Neostigmine is often still used due to its low cost compared with sugammadex.
Dosage & Indications
For muscular relaxation during non-emergent endotracheal intubation.
Intravenous dosage
Adults
0.45 to 1.2 mg/kg/dose IV. Onset of intubating conditions is less than 2 minutes. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.
Infants, Children, and Adolescents
0.45 to 0.6 mg/kg/dose IV. Onset of intubating conditions is 60 to 75 seconds.[42031] A lower dose of 0.3 mg/kg IV has been used successfully in combination with inhalation anesthesia induction for surgery.[52549] Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.[42031]
Neonates
0.45 to 0.6 mg/kg/dose IV. Onset of intubating conditions is 1 to 2 minutes.[42031] [44872] [53140] [53151] The lower dose of 0.45 mg/kg results in significantly shorter recovery time compared to larger doses.[53151] Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.[42031]
For muscular relaxation during rapid-sequence intubation (RSI).
Intravenous dosage
Adults
0.6 to 1.2 mg/kg/dose IV. Onset of intubating conditions is less than 2 minutes. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.
Infants†, Children†, and Adolescents†
0.6 to 1.2 mg/kg/dose IV. Usual dose: 1 mg/kg/dose. Onset of intubating conditions is 1 to 2 minutes.[44771] [52550] [64934] Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.[42031]
Neonates†
0.45 to 1.2 mg/kg/dose IV. Onset of intubating conditions is 1 to 2 minutes.[44872] [53140] [53151] Specific recommendations for RSI are not available; doses are extrapolated from use in non-emergent situations. The lower dose of 0.45 mg/kg results in significantly shorter recovery time compared to larger doses.[53151] Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.[42031]
For neuromuscular blockade during mechanical ventilation in intensive care patients.
Intermittent Intravenous dosage
Adults
0.6 to 1 mg/kg IV once, followed by 0.1 to 1 mg/kg/dose IV as needed; adjust dose and interval to patient’s twitch response. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.
Infants, Children, and Adolescents
0.45 to 0.6 mg/kg IV once, followed by 0.075 to 0.6 mg/kg/dose IV as needed; adjust dose and interval to patient’s twitch response. Children (3 to 11 years) generally have the largest dosage requirement. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.
Neonates
0.45 to 0.6 mg/kg IV once, followed by 0.075 to 0.6 mg/kg/dose IV as needed; adjust dose and interval to patient’s twitch response. Neonates generally have a lower dosage requirement. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.
Continuous Intravenous Infusion dosage
Adults
0.6 to 1 mg/kg IV bolus, followed by 8 to 12 mcg/kg/minute continuous IV infusion; titrate to patient’s twitch response. Usual dosage range: 4 to 16 mcg/kg/minute. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.
Infants, Children, and Adolescents
0.6 mg/kg IV bolus, followed by 5 to 10 mcg/kg/minute continuous IV infusion; titrate to patient’s twitch response. Children (3 to 11 years) generally have the largest dosage requirement. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.
Neonates
0.6 mg/kg IV bolus, followed by 5 to 10 mcg/kg/minute continuous IV infusion; titrate to patient’s twitch response. Neonates generally have a lower dosage requirement. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.
For neuromuscular blockade during surgery.
Intermittent Intravenous dosage
Adults
0.45 to 1.2 mg/kg IV once, followed by 0.1 to 0.2 mg/kg/dose IV as needed; adjust dose and interval to patient’s twitch response. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.
Infants, Children, and Adolescents
0.45 to 0.6 mg/kg IV once, followed by 0.075 to 0.15 mg/kg/dose IV as needed; adjust dose and interval to patient’s twitch response. Children (3 to 11 years) generally have the largest dosage requirement. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.[42031]
Neonates
0.45 to 0.6 mg/kg IV once, followed by 0.075 to 0.15 mg/kg/dose IV as needed; adjust dose and interval to patient’s twitch response. Neonates generally have a lower dosage requirement. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.[42031]
Continuous Intravenous Infusion dosage
Adults
0.45 to 1.2 mg/kg IV bolus, followed by 10 to 12 mcg/kg/minute continuous IV infusion; titrate to patient’s twitch response. Dosage range: 4 to 16 mcg/kg/minute. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.
Infants, Children, and Adolescents
0.45 to 0.6 mg/kg IV bolus, followed by 7 to 12 mcg/kg/minute continuous IV infusion; titrate to patient’s twitch response. Children (3 to 11 years) generally have the largest dosage requirement. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.[42031]
Neonates
0.45 to 0.6 mg/kg IV bolus, followed by 7 to 12 mcg/kg/minute continuous IV infusion; titrate to patient’s twitch response. Neonates generally have a lower dosage requirement. Coadministration of certain drugs may need to be avoided or dosage adjustments may be necessary; review drug interactions.[42031]
For the prevention of shaking chills† induced by therapeutic hypothermia after cardiac arrest.
Intermittent Intravenous dosage
Adults
Limited data; 0.6 mg/kg/dose IV as needed.[56387] Guidelines suggest that neuromuscular blocking agents may be used to manage overt shivering in therapeutic hypothermia.
Continuous Intravenous Infusion dosage
Adults
Limited data; 0.25 mg/kg IV bolus, followed by 0.25 mg/kg/hour continuous IV infusion or 0.6 mg/kg IV bolus, followed by 0.15 mg/kg/hour continuous IV infusion.[56387] [65372] Guidelines suggest that neuromuscular blocking agents may be used to manage overt shivering in therapeutic hypothermia.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.
External Link
Wikipedia contributors. (2020, July 19). Rocuronium bromide. In Wikipedia, The Free Encyclopedia. Retrieved 22:43, November 24, 2020, from https://en.wikipedia.org/w/index.php?title=Rocuronium_bromide&oldid=968388831